
Light emitted or absorbed by single atoms contributes only very little to the colours of our surroundings. The same file with adapted formatting can be found here. Both qualitative and quantitative spark analysis are widely used for production quality control in foundry and metal casting facilities.Atomic spectra The html formatting and custom instructions have been disabled on this server. However, modern spark sources with controlled discharges can be considered quantitative. In the past, the spark or arc conditions were typically not well controlled, the analysis for the elements in the sample were qualitative. The excited analyte atoms emit light at characteristic wavelengths that can be dispersed with a monochromator and detected. An electric arc or spark is passed through the sample, heating it to a high temperature to excite the atoms within it. In traditional arc spectroscopy methods, a sample of the solid was commonly ground up and destroyed during analysis. For non-conductive materials, the sample is ground with graphite powder to make it conductive. Spark or arc atomic emission spectroscopy is used for the analysis of metallic elements in solid samples. These procedures include incorporating electrothermal vaporization, laser and spark ablation, and glow-discharge vaporization. With plasma emission, it is possible to analyze solid samples directly. Larger water droplets condense on the sides of the spray chamber and are removed via the drain, while finer water droplets move with the argon flow and enter the plasma. Then the samples pass through a nebulizer that creates a fine mist of liquid particles. Liquid samples are pumped into the nebulizer and sample chamber via a peristaltic pump. The predominant form of sample matrix in ICP-AES today is a liquid sample: acidified water or solids digested into aqueous forms. Because plasmas operate at much higher temperatures than flames, they provide better atomization and a higher population of excited states. Plasma's high-temperature results from resistive heating as the charged particles move through the gas. The plasmas used in atomic emissions are formed by ionizing a flowing stream of argon gas. Plasma is a collection of charged particles (cations and electrons) capable, by virtue of their charge, of interacting with a magnetic field. This current induces a magnetic field inside the coil, coupling a great deal of energy to plasma contained in a quartz tube inside the coil. An induction coil is a coil of wire that has an alternating current flowing through it. Inductively coupled plasma (ICP) source of the emission consists of an induction coil and plasma. Disadvantages are spectral interferences (many emission lines), cost and operating expense and the fact that samples typically must be in a liquid solution. Īdvantages of ICP-AES are the excellent limit of detection and linear dynamic range, multi-element capability, low chemical interference and a stable and reproducible signal.
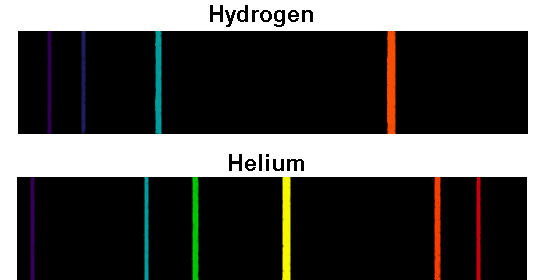
Inductively coupled plasma atomic emission spectroscopy (ICP-AES) uses an inductively coupled plasma to produce excited atoms and ions that emit electromagnetic radiation at wavelengths characteristic of a particular element. Inductively coupled plasma atomic emission source


 0 kommentar(er)
0 kommentar(er)
